SUB-STUDIES
Adding value to clinical research;
the ASPREE project is in a unique position to lead multiple sub-studies, each investigating specific health issues affecting older adults.
What is a sub-study?
→ View ASPREE sub-studies
A sub-study (also known as an ancillary study) is a type of ‘add-on’ study that answers a separate research question within a larger research project. Sub-studies research specific health questions that are not included in the main study. The ASPREE project is in a unique position to conduct multiple sub-studies. The extra health information will help show the interrelationships between various factors that can affect how we age.
Sub-studies provide new opportunities and experiences for participants – from donating blood samples, to having hearing tests, retinal photographs, sleep apnoea checks, MRI scans, to completing additional questionnaires about lifestyle, diet and more. Not all ASPREE participants were able to enrol in all sub-studies because each sub-study was introduced at a different stage in the trial, when funding became available or special equipment was present at the time. However, most participants volunteered for at least one sub-study during the ASPREE trial.
Clinically significant findings, i.e. findings important for a person’s health care, which were detected during sub-study activities, were referred to the participant’s GP for follow-up care.
Some sub-studies use existing medical health records and do not require any additional participant activities.
Thanks to the generosity of our study participants, these sub-studies provide invaluable information about aspirin, health and ageing, that we would not otherwise be able to collect in the main research project.
ASPREE sub-studies
Click on the sub-study below for more information

Above: Inside the ASPREE-AMD RetCam Van.
ASPREE – AMD
A study of low-dose aspirin and age-related macular degeneration
Summary: Age-related macular degeneration (AMD) is a degenerative disease affecting the middle area of the retina, called the macula, at the back of the eye. AMD is the leading cause of vision loss in older adults. While the cause is unknown, risk factors include a family history of AMD, smoking and being older than 50 years of age. Symptoms include blurred or distorted vision, reduced central vision and increasing difficulty reading small print (even when wearing glasses). There are two forms of AMD:
- Dry – develops slowly and results in gradual deterioration of vision (most common)
- Wet – develops rapidly and is due to bleeding beneath the retina
Aims: To investigate the effect of daily low-dose aspirin on AMD. Aspirin may be of benefit in the early stages of AMD, but not the later stages of the disease. However, no one really knows because previous research has been inconsistent or statistically insignificant.
Health Information Collected:
- Retinal imaging (photographs of the back of the eye) at enrolment in the ASPREE trial and repeated three and five years following enrolment
- Three vehicles fitted with specialised imaging equipment (RetCam Vans) enabled participants living within metropolitan and greater Melbourne, in regional Victoria, Hobart and southern NSW to contribute retinal images to this sub-study (pictured above).
Location: Australia
Status: Active, not recruiting. Commenced 2013, with collection of five year images concluding in 2021
Additional information: AMD Fact sheet
Chief Investigator: Prof John McNeil
Publications: Yes
Collaborators:
- Monash University
- The Centre for Eye Research Australia (CERA)
- University of Iowa (USA)
- GP Associate Investigators
Funding:
- National Eye Institute (of the National Institutes of Health, USA)
- The Australian National Health and Medical Research Council (NHMRC) project grant 2013-2017
- The Phyllis Connor Memorial Trust managed by Norman Bourke and Equity Trustees funded the first RetCam Van
- The Jack Brockhoff Foundation grant for the purchase of a retinal image camera
- The Eric Ormond Baker Foundation grant awarded to CERA towards the purchase of a retinal imaging camera
- NHMRC grant awarded to CERA towards the purchase a retinal camera for ASPREE-AMD
- Monash University funded 2 further RetCam Vans
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 10.05.2023
ASPREE-Anaemia
A study of low-dose aspirin and anaemia in older adults
Above: An illustration of red blood cells in our blood. Red blood cells contain haemoglobin to transport oxygen around our body.
Summary: Anaemia is the term for low levels of haemoglobin (Hb), a protein in the blood which carries oxygen around the body. Although anaemia increases with age, in approximately 30% of cases of anaemia in older adults the cause is unknown. There have been few studies into the effect that persistent anaemia has on the health and wellbeing of older adults.
All ASPREE participants had normal levels of haemoglobin when they enrolled in the ASPREE clinical trial and underwent annual Hb measures. ASPREE-Anaemia will investigate how frequently healthy older adults develop anaemia, the causes and its effect on quality of life. It will also determine the effect of aspirin on the development of anaemia in this age group.
Aims:
- To understand how often and why some well older adults become anaemic while others do not
- Measure the effects of low-dose aspirin on the development of anaemia and Hb levels
- To determine the significance of anaemia on mortality (lifespan), cognitive (thinking and memory) and physical function, and quality of life
Health Information Collected:
- A range of biomarkers (such as certain proteins, vitamins, hormones and indicators of inflammation) in blood samples donated by 12,000 participants at enrolment into the ASPREE Healthy Ageing Biobank and in more than 10,000 participants’ follow-up blood samples taken three years later
- Clinical and pathology measures and health outcomes, such as hospitalisation data collected in the ASPREE clinical trial
Location: Australia
Status: Active, not recruiting. Initial analysis completed.
Chief Investigators: A/Prof Zoe McQuilten, Prof Erica Wood
Publications: Yes
Collaborators:
- Monash University
- GP Associate Investigators
Funding:
- Alfred Research Trusts
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 05.07.2023
ASPREE ANTI-Sepsis (AspiriN To Inhibit SEPSIS)
A study of low-dose aspirin and severe infection

Above: The ASPREE Anti-Sepsis sub-study collected information about hospitalisations for severe infections.
Summary: Sepsis is a very serious and potentially life-threatening condition caused by the body’s response to severe infection. Studies indicate that aspirin may help reduce the severity of inflammation associated with infection and may subsequently reduce the severity of sepsis. However, this has not been proven.
Aims: The ASPREE ANTI-sepsis sub-study will determine whether low-dose aspirin:
- reduces the number of severe infection episodes requiring hospital admissions
- reduces ICU (Intensive Care Unit) admissions among patients hospitalised for severe sepsis
- reduces the mortality rate of sepsis in older people
Health Information Collected:
- Health information about hospitalisation and infection are collected entirely through the participant’s medical records
Location: Australia
Status: Completed, with findings published
Chief Investigator: Prof Damon Eisen
Publications: Yes
Collaborators:
- Monash University
- The Victorian Infectious Diseases Service
- Melbourne Health
- Alfred Health
- GP Associate Investigators
Funding
- The Australian National Health and Medical Research Council
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 09.08.2021
ASPREE Atrial Fibrillation (AF)
A study of atrial fibrillation and cognition
Above: An illustration of the outside and inside of the heart. Heart muscle usually contracts (beats) in a regular rhythm.
Summary: Atrial fibrillation (AF) is a common heart rhythm disorder causing the heart to beat irregularly and often fast. The risk of AF increases with age and it is one of the leading causes of stroke.
AF is associated with impaired cognition (thinking and memory) and dementia in older adults, even without evidence of a stroke. It is unknown whether a decline in thinking and memory may be due to changes in the small blood vessels in the brain, or due to small silent strokes.
There is currently a lack of research to understand how AF may affect the structure of blood vessels and tissue in the brain and how this impacts thinking and memory in older adults.
Aims: To investigate and understand the effect of atrial fibrillation on cognitive impairment and dementia
Health Information Collected:
- This sub-study is undertaken entirely through examination of medical records and health information collected during the ASPREE trial and ASPREE-XT study and brain imaging and cognitive data collected in ASPREE-Neuro, SNORE-ASA and ENVIS-ion sub-studies
- Participant health outcomes (endpoints), such as dementia, are adjudicated by panels of clinical specialists
Location: Australia
Status: Active, not recruiting. Commenced in 2019, data collection continues
Chief Investigator: Prof Amy Brodtmann
Publications: N/A
Collaborators:
- Monash University
- Berman Center for Outcomes and Clinical Research
- Florey Institute
- University of Minnesota, Health Heart Care
- University of Melbourne
- GP Associate Investigators
Funding
- NHMRC – The Australian National Health and Medical Research Council
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 09.08.2021
ASPREE Biobank
Biospecimens from older adults

Above: Biospecimens donated to the ASPREE Biobank are being prepared for long-term storage at ultra low temperatures in the Monash Department of Epidemiology Biorepository.
Summary:
The ASPREE Biobank is the first of its kind to store blood and urine samples from more than 12,000 healthy Australian adults aged 70+. Each sample is associated with a wealth of information about the donor’s health at the time of collection and into the future. This enables researchers to compare bio-specimens from those who develop older onset disease such as dementia and cancer, with those who do not.
Samples collected from the same person, at different time points, enables researchers to track changes in biomarker levels (such as protein) in the blood or urine, which may be associated with disease or even good health. The ASPREE Biobank has already supported a number of sub-studies such as the SHOW study (Sex Hormones in Older Women) and ASPREE-G (Genomics).
All biobank samples are de-identified and barcoded for long-term storage. The blood is separated into various components – white blood cells, red blood cells, plasma or serum – and stored in multiple tiny tubes (aliquots) at ultra-low temperatures; all within four hours of the donation. Urine samples are similarly stored in tiny tubes at very low temperatures.
Collection of a third wave of blood and urine samples from ASPREE-XT participants is underway. These latest samples will contribute to future biobank studies as well as support the ASPREE-XT Microbiome study. Samples from the biobank and microbiome studies enables research into how the gut microbiome and their metabolites (by-products), which are absorbed into the blood, affect health and diseases, such as dementia.
Participants do not need to have donated samples to the biobank previously, or be in the ASPREE-XT Microbiome study to take part in the ASPREE biobank. Our sincere thanks to Biobank participants for making this world-leading resource possible – every contribution supports research needed to improve health and wellbeing in older age.
Aims: Create a unique resource of biospecimens for vital research that aims to improve health and ageing well for older adults.
Samples collected:
- Blood or saliva and urine samples collected from more than 12,000 participants prior to commencing ASPREE tablets (aspirin or a placebo), or within the first 12 months of being in the trial
- A follow-up sample collected three years later from more than 10,500 of participants who gave an initial sample
- Collection of a third wave of blood and urine samples for the Biobank (open to all ASPREE-XT participants) commenced in 2021
Location: Australia
Status: Active, recruiting
Additional information:
Chief Investigators: Prof John McNeil and A/Prof Robyn Woods
Publications: Manuscript in progress
Collaborators (baseline & three year follow-up):
- Monash University
- Australian National University
- Menzies Institute for Medical Research (Tasmania)
- Queen Elizabeth Hospital/University of Adelaide
- University of Melbourne
- St John of God Pathology
- Healthscope Pathology (now known as Clinical Labs Pathology)
Collaborators (from 2021 onwards):
- Monash University
- Australian National University
- Menzies Institute for Medical Research (Tasmania)
- University of Adelaide
- MGH/Harvard University
- Berman Center for Outcomes & Clinical Research
- Clinical Labs Pathology
Funding (baseline & three year follow-up)
- Commonwealth Scientific and Industrial Research Organisation (CSIRO) (initial samples)
- Monash University
- NHMRC – The Australian National Health and Medical Research Council
- Victorian Cancer Agency (VCA)
- National Cancer Institute (NCI) (follow up samples), USA
- Walter Cottman Endowment Fund and Equity Trustees (Biobus)
Funding (from 2021 onwards)
- National Institutes on Aging (NIA/NIH), USA
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 10.05.23
ASPREE Cancer Endpoints Study (ACES)
A study of cancer in older adults
Above: An illustration of a cancer tissue sample in preparation for close examination by researchers
Summary: The ACES sub-study is funded by the National Cancer Institute (NCI), with the aim to understand factors associated with the development and spread of different types of cancers in older adults.
Cancer is one of the most common diseases and a leading cause of death worldwide. Ageing is a factor that increases the risk of cancer.
ACES collects cancer related health information from participants annually and stores blood or saliva and cancer tissue samples (biospecimens) in a biorepository.
Aims: Research using these data may help predict what factors influence the incidence of specific cancers, their spread (metastasis) and the impact of a therapy (including low-dose aspirin) on cancer-free survival.
Health Information collected:
- Participant reported lifestyle factors such as smoking and drinking history, body weight, cancer screening, tests, cancer diagnoses (old and new) and family history of cancer are collected annually
- Medical/specialist health records related to a cancer diagnosis
- A sample of blood or saliva is stored in the Healthy Ageing Biobank
- A tumour tissue sample – If a participant has a cancer biopsied or surgically removed as part of their doctor’s care, we collect a small sample of the tumour tissue from the pathology provider, for storage in our biorepository
Location: Australia and USA
Status: Active, not recruiting. Commenced in 2013, data collection continues
Additional Information: Participant FAQs
Chief Investigator: Dr Suzanne Orchard
Publications: Yes
Collaborators:
- Monash University
- Berman Center for Outcomes and Clinical Research (Minneapolis, MN) plus 22 centres across the USA
- Menzies Institute of Medical Research (TAS)
- The University of Melbourne
- Australian National University
- The University of Adelaide
- GP Associate Investigators
Funding:
- National Cancer Institute (NCI), USA
- Monash University
- Commonwealth Scientific and Industrial Research Organisation (CSIRO)
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 09.08.2021
ASPREE Cancer Treatment Study
A study of cancer treatment in older adults

The ASPREE Cancer Treatment will determine whether surviving cancer increases the risk of persistent health problems for older adults.
Summary: Cancer is one of the most common diseases and a leading cause of death worldwide. Ageing increases the risk of cancer.
Early detection and new treatments have generally increased cancer survival. Research suggests that cancer survivors are more likely to develop persistent health problems and experience more rapid declines in cognition (thinking and memory) and functional ability than similar-aged adults who have not had cancer. It is not well understood if these increased risks are due to cancer itself, or whether the treatment, overall, or specific treatment type, plays a role.
Aim: To determine whether surviving cancer increases the risk of persistent diseases, such as cardiovascular disease, cognitive health and physical function.
Health Information collected:
- This sub-study is undertaken entirely through examination of medical records and health information
- Involves the collection of medical/specialist health records related to cancer treatments that ASPREE participants undertake after being diagnosed with cancer, including surgery details and therapies (such as chemotherapy and radiation)
Location: Australia and USA
Status: Active, not recruiting. Commenced in 2020, data collection continues
Chief Investigators: Prof John Zalcberg, Dr Suzanne Orchard
Publications: N/A
Collaborators:
- Massachusetts General Hospital/Harvard University
- Monash University
- Berman Center for Outcomes and Clinical Research (Minneapolis, MN) plus 22 centres across the USA
- GP Associate Investigators
Funding:
- NIA – National Institute on Aging, USA
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 09.08.2021
ASPREE-CHIP study
A study of clonal haematopoiesis of indeterminate potential

It is unknown what proportion of older adults have Clonal Haematopoiesis of Indeterminate Potential (CHIP).
Summary: Clonal haematopoiesis is the presence of a unique age-related genetic variation (mutation) found in blood cells and cells in the bone marrow (where the blood cells form). This genetic variation is not inherited, but develops over a period of time in some older adults. Clonal haematopoiesis of indeterminate potential (CHIP) is the presence of this genetic variation in the blood, without the person showing any signs or symptoms of a blood disorder.
Recent studies have shown that CHIP is increasingly common with age and is associated not only with increased risk of blood cancers but also cardiovascular disease (heart attack and stroke). It is thought that inflammation may have a role in whether CHIP influences the development of cardiovascular disease in some older adults and not others.
Aims:
- To identify the proportion of healthy older adults with CHIP
- Determine the associations between CHIP and blood disorders, coronary heart disease (diseased blood vessels of the heart) and ischaemic stroke (stroke caused by blood clots) in older adults
- Determine whether aspirin’s anti-inflammatory properties affects the incidence, progression and effects of CHIP on health
Health Information collected:
- Blood samples from 11,133 participants at enrolment into the ASPREE Healthy Ageing Biobank and three year follow-up samples from 10,400 participants to measure CHIP and inflammatory biomarkers at two time-points
- Health outcomes of haematological (blood) cancers, coronary heart disease and ischaemic stroke in participants in the aspirin arm and the placebo arm of the ASPREE clinical trial
Location: Australia
Status: Active, not recruiting. Commenced 2021, data collection continues.
Chief Investigator: A/Prof Zoe McQuilten
Publications: N/A
Collaborators:
- Monash University
Funding:
- Monash University
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 09.08.2021
ASPREE-D (Depression)
A study of low-dose aspirin and depression in older adults

ASPREE-D was the first to study the effect of aspirin versus placebo on the prevention of depression in older adults.
Summary: An estimated 10-15% of people aged between 70 – 85 years will experience depression at some point in time. Symptoms of depression are often missed and untreated and can be mistakenly attributed to ageing and subsequently remain undetected and untreated. In older people, untreated depression is associated with a wide range of poor health outcomes, including an increased risk of dementia, social isolation, loss of quality of life and a shortened lifespan. Depression is associated with increased inflammation raising the question whether anti-inflammatory therapies might be useful for prevention or treatment of depression.
Aim: Aspirin has well-known anti-inflammatory actions. With the large number of people enrolled in the ASPREE trial, for the first time, researchers in the ASPREE-D sub-study investigated whether aspirin prevented the onset of depression in older adults, or could alleviate depression in those presenting with depression.
Health Information Collected:
- ASPREE participants answered questions about their history of depression and completed a measure of depression called the Center for Epidemiologic Studies Depression Scale (CES-D)-10 item scale at annual study visits. The (CES-D)-10 is a screening tool that may indicate possible depression. While only a qualified medical practitioner can make a formal diagnosis, the CESD is a reasonable proxy for diagnosis.
- Researchers will also examine blood samples in the ASPREE Healthy Ageing Biobank for the presence (or absence) of proteins or ‘biomarkers’ associated with inflammation. They will then be able to determine the relationship between these inflammatory ‘biomarkers’ in the blood and diagnosed depression. Knowing which biomarkers are associated with depression may help predict those at risk of depression or may help identify people likely to benefit from aspirin.
Location: Australia and USA
Status: Completed. Main findings published (with exception of the inflammatory biomarker component)
Chief Investigator: Prof Michael Berk
Publications: Yes
Collaborators:
- Monash University (Australia)
- Deakin University, School of Medicine (Australia)
- Rush University Medical Center, Department of Family Medicine and the Rush Alzheimer’s Disease Center (USA)
- University of Melbourne, Department of Psychiatry and School of Public Health and Preventive Medicine (Australia)
- Orygen Youth Health Research Centre (Australia)
- Erasmus Medical Center, Department of Immunology (The Netherlands)
- Florey Institute of Neuroscience and Mental Health (Australia)
- GP Associate Investigators
Funding:
- The Australian National Health and Medical Research Council (NHMRC) grant 1081901
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 08.09.2022
ASPREE-EWAS (Epigenetics)
A study of blood DNA methylation in older adults
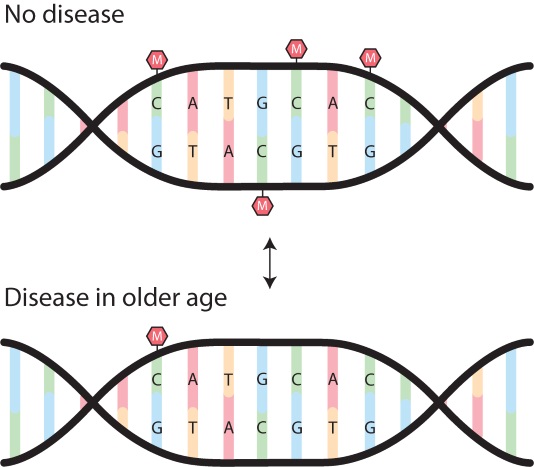
Above: An illustration of a methylation (M) pattern on our genes. The top picture represents the gene acting normally, when we are in good health. Methyl groups can act as a ‘stop’ sign to genes associated with disease. In the lower image, the methyl groups are removed and the genes associated with a disease are switched ‘on’.
The EWAS sub-study will examine how lifestyle and environmental factors, such as nutrition, physical activity, pollution and stress may change DNA methylation to switch gene function ‘on’ or ‘off’ over time.
Summary: We are all born with our own individual DNA or genetic code. The genetic code is the body’s blueprint, containing all the necessary information for us to grow and function. Generally, our genetic code remains relatively fixed from conception until death. However additional information in our cells helps control how our genetic code is used over a lifetime, such as when the function of a gene is switched ‘off’ or ‘on’.
Lifestyle and environmental factors, such as nutrition, physical activity, pollution and stress may trigger one of several epigenetic processes which can activate or deactivate gene function. For instance, good nutrition may influence the production of chemicals (methylation) which attach to the genetic code and ‘turn-off’ genes associated with heart problems. This explains how a good diet may affect heart health at a genetic level.
Epigenetics is still a relatively new field and much more research is needed to understand how lifestyle behaviours such as increasing exercise may alter or reverse epigenetic patterns over the short and long term.
Epigenetic changes are also thought to underlie certain diseases. Some of the most widely studied mechanisms of epigenetic regulation include DNA methylation (the attachment of a chemical to the genome), histone modifications and non-coding RNAs. Due to its stability and ease of measurement, DNA methylation is most commonly examined in large studies involving many individuals.
This sub-study measures epigenome-wide DNA methylation patterns (EWAS) in blood samples donated by ASPREE participants at enrolment into the trial and three years later.
Researchers will examine 850,000 sites for biomarkers across all genes. The DNA methylation biomarkers can be matched with participants’ lifestyle, behaviours and health at the time the sample was given, and prior to future health events, such as cancer or depression, should they occur.
The EWAS sub-study will enable researchers to use DNA methylation biomarkers to help identify older adults at risk of developing health conditions and help identify factors which may prevent or progress poor health.
Aims:
- Determine the DNA methylation patterns of individuals in the ASPREE Healthy Ageing Biobank at baseline and three-years, and contribute to ASPREE’s research interests in cancer, dementia, cardiovascular disease, physical disability, depression and frailty and other conditions affecting health as we age
- Investigate how lifestyle and behaviours are associated with DNA methylation patterns and changes in patterns over time
Health Information Collected:
- Blood samples provided to the ASPREE Healthy Ageing Biobank, at study recruitment and at three-years
- Relevant health, lifestyle and behaviour information provided by ASPREE participants during the course of the ASPREE trial and follow up ASPREE-XT study
- Participant health outcomes (endpoints)
Location: Australia and USA
Status: Active, not recruiting
Chief Investigator: A/Prof Joanne Ryan
Publications: Yes
Collaborators:
- Monash University (Australia)
- Berman Center for Outcomes and Clinical Research
- GP Associate Investigators
Funding:
- Monash University
- NHMRC – The Australian National Health and Medical Research Council
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 09.08.2021
End of Life Sub-study

Summary: The changing nature of medical care at the end of life is of increasing international interest. Rapid advances of medical technology, an ageing population across the world and stretched resources within the clinical workforce, as well as changing social norms, have brought end of life care into the fore.
There is currently very little information about the quality and nature of end of life care in Australian communities and hospitals, with most information coming from UK studies.
How and when do older adults, after a long healthy lifespan, discuss end of life choices? What is the reality of end of life care?
We don’t know because detailed questions have not been asked by Australian researchers before.
This sub-study collects diverse information about end of life care, including symptom control (such as pain relief), end of life choices, and communications and support. Findings will help inform and improve health services and support for older Australians and their loved ones.
Aims:
- To interview the closest contact of ASPREE participants who have died within the last 24 months to understand the quality and circumstance of the death from a broad cross-section of the Australian community.
- Data is collected via a 32-question survey, which may be answered by phone, email or mail.
Information Collected Includes:
- Mobility and transport
- Quality of symptom control and relief
- Quality of communication and access to medical care
- Satisfaction with medical care provided for older Australians at end of life
- Whether participants and families had prior knowledge or warning of the dying process, and if they had adequate time to prepare for end of life
- Treatment preferences organised by participants towards end of life
Location: Australia
Status: Active, not recruiting
Contact: Ms Cammie Tran
Publications: No
Collaborators:
- Monash University (Australia)
Funding:
- Monash University
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 7 February 2022
ASPREE – Fracture
A study of low-dose aspirin and prevention of falls and fractures

The ASPREE – Fracture sub-study examines whether aspirin reduces the risk of bone fracture in older adults.
Summary: Weakened and brittle bones contribute to an estimated 25,000 fractures that occur each day around the world. Bones tend to become more fragile with age and research shows that more than 90% of hip fractures are the result of a fall. Results from previous studies have been inconsistent about the effect of aspirin on bone health.
Aims: The ASPREE-Fracture sub-study examined whether daily low-dose aspirin:
- reduced the risk of older Australians having a bone fracture
- reduced the risk of having a fall that resulted in hospital attendance
Health information collected: Information about fractures and presentations to hospital with a bone fracture was collected entirely through the participant’s medical records.
Location: Australia
Status: Completed
Chief Investigator: A/Prof Anna Barker
Publications: Yes
Collaborators:
- Monash University
- University of Melbourne
- Western Health
- Austin Health
- The Mayo Clinic, USA
- University of Sydney
- Deakin University
- GP Associate Investigators
Funding:
- NHMRC – The Australian National Health and Medical Research Council
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 16.11.2022
ASPREE-G (Genomics)
A study of genetics on the health of older adults

Above: An illustration of the structure of DNA.
Summary: Genetic research is a broad term for the study of traits passed down generations through the inheritance of genetic material (such as DNA). Genetic research has an important role in medical research.
Genes are the fundamental unit or ‘blue-print’ of life which can determine many of our physical traits such as eye colour and blood type. Genes also contribute, in combination with the environment, to more complex human traits such as personality, behaviour and the risk of developing a disease.
Humans have approximately 20,000 genes, each gene composed of a chemical called DNA (deoxyribonucleic acid). DNA gives the instructions or the chemical code to express traits, such as telling the body which type of protein to make and when. These proteins form part of a complex chain of events that impact on how we look and function.
Thanks to rapid advances in technology, an individual’s entire genetic information can be studied through a process called genome sequencing. Genome sequencing reveals more about human genetics than ever before; it also reveals large expanses of the human genome about which we know very little, if anything.
Our health often has at least a small genetic component. Longevity that runs in families is an example of how genes may influence good health. Genetic research is in many ways, pioneering. Genetic research in older adults aims to understand the role genetic make-up has on our health so that one day we may be able to better detect or prevent age-related diseases such as cancer, dementia and cardiovascular disease.
Aims:
Genetic studies on Biobank samples contribute to a growing body of research knowledge about genetics and health. Samples in the ASPREE Healthy Ageing Biobank will enable researchers to better understand the role of our genes in health and ageing. Discoveries may take time to emerge, however their benefits and impact will be highly important for many years to come.
Health information collected:
- More than 15,000 Australian and US ASPREE participants have generously donated blood or saliva samples for future biomarker or genetic research.
- Each sample contains DNA from healthy adults who have consented to genetic research (most other genetic studies look at the genes from people with disease)
- All samples are of very high quality, maximising research opportunities and are associated with unprecedented health and lifestyle information to help bridge the link between our health and what is happening at a genetic level
- Genetic studies on ASPREE Biobank samples are for research purposes only and are not intended to be medical investigations or diagnostic tests
Location: Australia and USA
Additional information: FAQs for ASPREE participants
Chief Investigator: A/Prof Paul Lacaze
Status: Active, not recruiting
Genomic projects:
1. The Medical Genome Reference Bank (MGRB) – involves sequencing of 4,000 genomes from healthy people aged 75 years and over from participants in the ASPREE study and the Sax Institute’s ’45 and Up’ Study. Genetic information from the genomes will be used to form a reference bank or library of ‘healthy genomes’ of the ‘wellderly’ (well older people). The ‘healthy’ reference bank can be used as a comparison by researchers to identify, more easily, genes that cause disease.
| MGRB Collaborators: | MGRB Funding: |
|
|
2. The Resilience Project – examines approximately 760 genes from ASPREE Biobank participants in Australia and the US for the presence of genetic variations associated with disease. The aim is to identify and understand why healthy older adults with these genetic variations remain free of disease when others do not. Additionally, the Resilience Project may also help reveal to ASPREE researchers genes that can reliably predict the onset of cardiovascular disease, cancer, dementia and depression in older people.
| The Resilience Project Collaborators: | The Resilience Project Funding: |
|
|
Publications: Yes
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 09.08.2021
ASPREE-Hearing
(Aspirin in HEAring Retinal vessels, Inflammatory markers, Neurocognition in older age Groups)

Summary: Hearing loss associated with ageing is experienced by two-thirds of people over the age of 70 years. Loss of hearing can have a profound effect on a person’s independence and quality of life. There is no known single cause and no known cure. The ASPREE-Hearing sub-study involved a series of hearing and cognitive measures, questionnaires and retinal images to help find the answer.
Aims:
- To investigate the role of low-dose aspirin on age-related hearing loss and the relationship between hearing loss and changes in cognition (thinking and memory).
Health information collected:
- Audiometry (hearing tests)
- Retinal imaging (photographs at the back of the eye)
- Cognitive (thinking and memory) measures
Location: Australia
Status: Completed
Additional information: ASPREE-Hearing Brochure
Chief Investigator: Prof John McNeil
Publications: Yes
Collaborators:
- Monash University
- National Acoustic Laboratories, Macquarie University
- University of Melbourne
- Johns Hopkins School of Public Health
- Menzies Institute for Medical Research (TAS)
- Centre for Eye Research Australia Ltd
- Monash Biomedical Imaging Centre (MBI)
- GP Associate Investigators
Funding:
- Monash University
- Deafness Foundation
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 09.08.2021
ASPREE Inflammatory Biomarkers
A study of inflammatory biomarkers in older adults
Above: Blood samples donated to the ASPREE Healthy Ageing Biobank enables researchers to investigate a range of inflammatory biomarker measures that may be linked to age-related diseases.
Summary: When inflammation occurs in the body, specific types of proteins called inflammatory biomarkers are often released from the affected part of the body into the bloodstream. These biomarkers indicate inflammation is happening generally, but they cannot pinpoint the cause of the inflammation.
Inflammation is thought to play a role in the cause and progression of a broad range of diseases and conditions, including depression. Doctors commonly check levels of inflammatory biomarkers in the blood to monitor inflammatory conditions, such as infections, autoimmune diseases and cancers. However, there is a lack of research into the importance and reliability of inflammatory biomarkers in ageing health.
This sub-study measures a range of inflammatory biomarkers in blood samples donated by ASPREE participants at enrolment into the trial and three years later. The biomarkers can be matched with participants’ health at the time the sample was given, and prior to future health events, such as cancer or depression, should they occur.
The inflammatory biomarker sub-study will enable researchers to use inflammatory biomarkers to help identify older adults at risk of developing health conditions and help identify factors which may prevent or progress poor health.
Aims: Provide a range of inflammatory biomarker measures that will contribute to ASPREE’s research interests in cancer, dementia, cardiovascular disease, physical disability, depression and frailty and other conditions affecting health as we age.
Health Information Collected:
- Inflammatory biomarkers in 22,354 blood samples donated by ASPREE trial participants to the ASPREE Healthy Ageing Biobank
- Relevant health information provided by ASPREE participants during the course of the ASPREE trial and follow up ASPREE-XT study
- Participant health outcomes (endpoints) as adjudicated by panels of clinical specialists
Location: Currently undertaken in Australian samples, and in 1500 US ASPREE participant samples in the future
Status: Active, not recruiting
Chief Investigator: A/Prof Robyn Woods
Publications: N/A
Collaborators:
- Monash University
- Deakin University, School of Medicine (Australia)
- University Medical Center Hamburg, Eppendorf, Hamburg, Germany
Funding:
- Monash University
- NHMRC – The Australian National Health and Medical Research Council
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 09.08.2021
ASPREE-Knee
A study of low-dose aspirin and osteoarthritis
Above: ASPREE-Knee participants underwent a knee MRI for this sub-study.
Summary: Osteoarthritis (OA) is a major cause of knee pain and knee replacement in older adults. The disease is characterised by a progressive thinning and loss of cartilage until the exposed ends of bones in a joint rub together causing pain. Currently, there is no treatment to reduce the loss of cartilage associated with OA.
It is thought that aspirin’s anti-inflammatory properties may help preserve cartilage in knee joints and reduce the burden of OA. However, the effect of aspirin on OA is unproven.
Aims: To understand whether the use of low dose aspirin slows the loss of knee cartilage in 170 older adults.
Health information collected:
- MRI of the knee to measure the amount of cartilage
- Physical activity questionnaire
- Recorded pedometer readings for seven days post MRI
Location: Melbourne, Australia
Status: Completed
Chief Investigator: A/Prof Anita Wluka
Publications: Data being collated in preparation for publication
Collaborators:
- The Musculo-skeletal Unit at the School of Public Health and Preventive Medicine, Monash University
- Monash University Biomedical Imaging Centre (MBI)
Funding:
- NHMRC – The Australian National Health and Medical Research Council
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page update: 09.08.2021
ASPREE Longitudinal Study of Older Persons (ALSOP) and the follow-up ALSOP-XT

Summary: ALSOP involves a series of questionnaires to help identify factors that may have a major influence on health, independence and quality of life as we grow older. For example, difficulties with hearing or eyesight, sleep, pain or falls are common problems for some older adults and can have a real impact on quality of life. Lifestyle and social factors, such as physical activity, access to transport and social connection are also important.
Following on from the ALSOP questionnaires completed by participants in Australia during the ASPREE trial, we are continuing to explore factors affecting the health, independence and quality of life of participants in a series of ALSOP-XT questionnaires being sent to ASPREE-XT participants in Australia and the USA.
Aim: To examine how general health, behavioural, social, economic and environmental circumstances affect health, quality of life and independence in older age.
Information collected:
A broad range of factors are explored including hearing, vision, dental health, pain, diet, physical activity, health service access, male and female health issues, social and community engagement, work, caring responsibilities, financial wellbeing and major life events.
ALSOP-XT builds on information provided by participants in ALSOP and includes a few additional questions related to the COVID-19 pandemic and women’s and men’s health.
Location: ALSOP was conducted in Australia. ALSOP-XT questionnaires are being sent to ASPREE-XT study participants in Australia and the USA in 2021, and repeated in 2023.
Status: Data collection continues. Baseline, year 3 and year 5 ALSOP questionnaires have been sent and returned. Medical ALSOP-XT questionnaires have been sent to Australian participants.
Additional information: Participant ALSOP postcard
Investigators:
- Prof John McNeil (Australia) for ALSOP questionnaires, years 1 – 5
- A/Prof Robyn Woods (Australia) Prof Andy Chan (USA) for the (post ASPREE) ALSOP-XT questionnaires
Publications: Yes
Collaborators:
ALSOP-XT
- Monash University
- Harvard University
- Berman Center for Outcomes and Clinical Research (Minneapolis, MN) plus 22 centres across the USA
Funding:
ALSOP
- Monash University
- The JO & JR Wicking Trust and Mason Foundation, ANZ Trustees
ALSOP-XT
- National Institutes of Health (NIH in the USA)
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 09.08.2021
ASPREE NEURO
Understanding the effect small changes in the brain may have on cognitive decline and stroke risk
Summary: Advanced imaging techniques have shown that with age, people commonly develop small changes inside their brains that have unknown impact on their health. Today’s MRI (Magnetic Resonance Imaging) scanners are frequently detecting tiny areas of bleeding ‘micro-haemorrhaging’ in the brain, blockages in small blood vessels and ‘white matter’ lesions (groups of cells) in the brains of otherwise healthy older adults.
Researchers do not know if these changes are possible indicators of potential stroke or dementia, or if they are a normal part of healthy ageing.
Aims: ASPREE-NEURO examines the relationship between the presence of white matter lesions and/or changes in small blood vessels of the brain and whether that puts older adults at risk of having a stroke or developing dementia in the future. MRI scans may also help to explain how aspirin affects these changes in the brain.
Health information collected:
- A total of three brain 3-tesla MRI scans: at enrolment into ASPREE (prior to commencing study tablets); one year later; and three years later
- A series of thinking and memory exercises, unless they had already been undertaken in the SNORE-ASA sub-study
Location: All MRI scans were undertaken at the Monash Biomedical Imaging Centre (MBI) Melbourne, Australia.
Status: Completed
Additional Information: NEURO brochure
Chief Investigator: Prof John McNeil
Publications: Yes
Collaborators:
- Monash University
- Monash University Biomedical Imaging Centre (MBI)
- GP Associate Investigators
Funding:
- Monash University
- NHMRC – The Australian National Health and Medical Research Council
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 09.08.2021
ASPREE-XT Microbiome Study
Studying gut microbiome and health in older adults
Above: An illustration of the variety of bacteria, viruses, fungi and other microbes in our gut.
Summary: There is increasing evidence that our gut microbiome can play a significant role in our health and wellbeing. The gut microbiome is a collective term for trillions of bacteria, viruses, fungi and other microbes in the gut and it can be measured in a stool or ‘poo’ sample.
Ageing is associated with changes in the gut microbiome, however these age-related changes in older adults are poorly understood. Previous studies have either been too small or limited to younger adults to understand the link between the types and number of microbes in the gut and its effect on good health and / or poor health in older persons
Aims:
- Understand the relationship between gut microbiome, diet, and lifestyle factors and how these may contribute to good health or the development of disease, including cancer.
- Identify the gut microbiome associated with cognitive decline and dementia
- Identify the gut microbiome associated with physical disability and frailty
Health information collected:
- A small stool scraping into three tubes, collected at home
- Tongue swab the following day, collected at home
- Two brief questionnaires; one about the stool sample, diet and medication use, and the other related to the tongue swab sample and dental health
- Information about diet and lifestyle factors reported in ALSOP-XT questionnaires (optional – see ALSOP sub-study for more information)
- Health events collected in the ASPREE-XT longitudinal study
- Blood and urine samples donated to the ASPREE Biobank (optional – see ASPREE Biobank for more information)
Microbial DNA will be extracted from the stool samples and used to identify the types and numbers of microbes to examine if there is a link between microbe content and good health or adverse health outcomes, such as cardiovascular disease, frailty or dementia.
Location: Australia and the USA
Status: Active, recruiting. Commenced in 2021, with health information collected at the time of the gut microbiome specimen collection.
Additional information:
Principal Investigators: A/Prof Robyn Woods (Australia) and Prof Andy Chan (USA)
Manager: Dr Alice Owen (Australia)
Publications: N/A
Collaborators:
- Monash University
- Harvard Medical School/ Massachusetts General Hospital
Funding:
- National Institute on Aging (NIA/NIH), USA
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 10.05.2023
Cognitive Follow-up study
Investigating the trajectory of memory and function decline among ASPREE participants

Summary: Understanding the effect that memory changes have on health and lifestyle as we age will assist with planning future health care service needs. This sub-study collects complementary information from an individuals’ relative or a close friend to better understand the impact that changes in thinking and memory may have on daily activities.
Aim: To investigate the way changes in thinking and memory can impact health and lifestyle as we age.
Health information collected:
With permission from eligible ASPREE participants, the study team call a nominated family member or friend for a brief series of questions about:
- Changes in the participant’s memory and its impact on their day to day activities, such as leaving the home alone and safely and managing medication
- Information is collected twice yearly during the ASPREE-XT study period
Location: Australia
Status: Active, not recruiting. Commenced 2020
Chief Investigator: Prof John McNeil
Publications: N/A
Collaborators:
- Monash University
Funding:
- NHMRC – The Australian National Health and Medical Research Council
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 05.12.2022
ENVIS-ion
Aspirin for the prevention of cognitive decline in the Elderly: a Neuro-Vascular Imaging Study of ASPREE
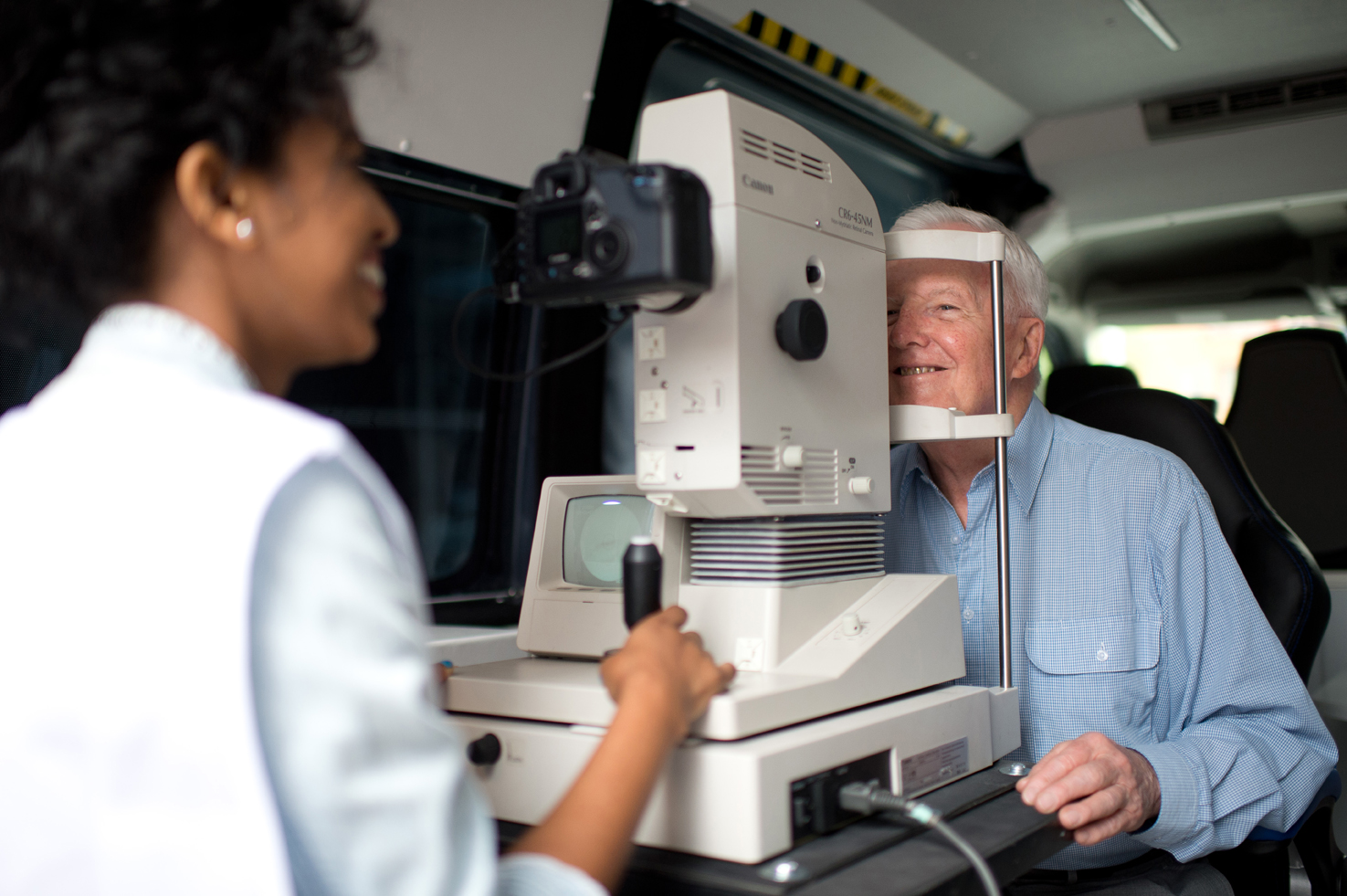
Above: Retinal photographs enables ENVIS-ion researchers to learn if eyes are windows to the brain
Summary: MRI (Magnetic Resonance Imaging) scans examine the small blood vessels in the brain and measure early changes in brain structure that are associated with the decline in thinking and memory. However, not everyone in the general population can undergo an MRI examination; the procedure is lengthy, quite expensive and cannot be undertaken in those with metallic implants, such as pacemakers.
Are eyes a window to the brain? It is thought that changes in the small blood vessels in the brain are also associated with changes in the small blood vessels in the back of the eye. Retinal photography is a quick and easy examination that takes about 5-10 minutes using a special digital camera.
Aims:
- To determine if changes in blood vessels in the eye correlate with changes in blood vessels in the brain. If so, retinal imaging may provide an easy and accessible window into brain health.
- To understand the effect of low-dose aspirin on age-related changes in the small blood vessels in the brain and eye and how these relate to thinking and memory or risk of stroke.
Health information collected: captured at enrolment into ASPREE (prior to commencing trial medication) and repeated 3 years later:
- MRI scans of blood vessels in the brain
- Thinking and memory exercises
- Retinal Images (photographs of small blood vessels in the back of the eye)
Location: Melbourne and Canberra, Australia
Status: Completed
Additional information: ENVIS-ion brochure
Chief Investigator: Prof Walter Abhayaratna
Publications: Yes
Collaborators:
- Monash University
- Australian National University: Department of Geriatric Medicine and the Clinical Trials Unit at the Canberra Hospital
- Alfred Health
- The Centre for Eye Research Australia (CERA)
- GP Associate Investigators
Funding:
- NHMRC – The Australian National Health and Medical Research Council
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 09.08.2021
SHOW
A study of Sex Hormones in Older Women

Summary: Cardiovascular disease (CVD; heart attack and stroke) is the leading cause of death in women aged 65 years and over. The risk of CVD increases steeply after midlife, implicating both age and possibly hormonal changes with ageing as playing a role.
The major sex hormones in women are oestrogens and androgens (such as testosterone). Oestrogen levels fall suddenly at menopause. Androgen levels do not change at menopause but start to fall from the mid 30’s, and then decline steadily with age until the 7th decade, after which testosterone levels start to increase.
There are some indications that low levels of testosterone may be associated with an increased risk of CVD in women, however this is still to be proven. Previous studies have been small, often conflicting and lacking the precision to accurately measure low concentrations of testosterone seen in older women.
Aims:
- Provide the first reference range for blood levels of the various androgens in women aged 70-74 years, 75-79 years, 80-84 years and 85+ years.
- Investigate the association between levels of androgens (testosterone and other androgens) in the blood and cardiovascular events and life expectancy in older women.
Health information collected:
- Androgen and oestrogen hormone levels in approximately 6,200 blood samples donated by female ASPREE participants to the Healthy Ageing Biobank shortly after they enrolled in the ASPREE trial (baseline) and in approximately 450 blood samples that had been provided to the biobank three years later.
- Health outcomes of Australian ASPREE participants, such as heart attack and stroke
Location: Australia
Status: Active, not recruiting
Chief Investigator: Prof Susan Davis, Women’s Health Research Program, Monash University
Publications: Yes
Collaborators:
- ANZAC Research Institute, University of Sydney
- GP Associate Investigators
Funding:
- NHMRC – The Australian National Health and Medical Research Council
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 09.08.2021
SNORE-ASA
Study of Neurocognitive Outcomes, Radiological and Retinal Effects of Aspirin in Sleep Apnoea

Above: How the portable sleep apnoea device was worn overnight in the SNORE-ASA sub-study.
Summary: Sleep apnoea refers to breathing problems during sleep. It becomes very common as we get older, and many people are not aware they have it, although in some people it may cause sleepiness in the daytime. Severe sleep apnoea may also increase the risk of some health conditions, such as stroke. Sleep apnoea may even affect the health of the brain overall, and lead to some changes in memory and thinking. Some of the ways that this may occur is through affecting the health of the small blood vessels in the brain. This study also examines whether in the setting of sleep apnoea aspirin can help slow down these associated changes in small blood vessels, and has a protective effect on memory and thinking changes in people with sleep apnoea.
Aims:
- To examine the long-term effects of severe sleep apnoea on cognitive outcomes in older adults
- To determine whether cognitive changes arise from damage to small vessels in the brain, and whether this progression is modified by low-dose aspirin therapy
Health information collected:
- Overnight apnoea monitoring via a portable device (as pictured above)
- Questionnaires about sleep quality and sleepiness
- Thinking and memory exercises at enrolment into ASPREE (and prior to taking trial tablet – 100 mg aspirin or matched placebo) and repeated after 3 years
- A sub-group of SNORE-ASA participants additionally underwent a brain MRI and/or retinal imaging
Location: Australia
Status: Completed
Additional information: SNORE-ASA Brochure
Chief Investigator: Dr Stephanie Ward
Publications: Yes
Collaborators:
- Monash University
- Alfred Health
- The Centre for Eye Research Australia (CERA)
- University of Queensland
- Austin Health
- GP Associate Investigators
Funding:
- NHMRC – The Australian National Health and Medical Research Council
Data Access: Contact the relevant sub-study chief investigator or visit the ASPREE Access Management Site (AMS) before submitting an expression of interest to access sub-study data.
Page updated: 09.08.2021
LATEST NEWS
Walking for transport at least once a week may help some older people live longer: ASPREE study
A new analysis of ASPREE data found that older adults who walked for transport instead of taking a car, at least once a week, lived longer than those who didn’t. This observational study in older adults looked at transport-related walking, which is walking for a specific purpose, such as to a medical appointment or to shop, instead of using motorised transport. Researchers found walking for transport at least once a week linked to a 25% lower risk of mortality compared to those who never walked for transport purposes.
Leisure activities that may reduce dementia risk – ASPREE analysis
In a study of leisure activities undertaken by ASPREE participants, using computers, writing, and...
Aspirin and anaemia risk in older adults
A new analysis of ASPREE trial data has found that prolonged daily aspirin use increases the risk of anaemia in some older adults. Findings from ASPREE-Anaemia, a sub-study of the ASPREE trial, may help GPs identify older patients at higher risk of anaemia and who may benefit from regular monitoring for development of the condition.







