FOR PARTICIPANTS
ASPREE
- The ASPREE Clinical Trial (completed)
- The follow-up ASPREE-XT Study (current)
→ Update your details
→ About Participants
→ FAQs, newsletters and more
→ Latest news
→ Contact us
Based on quality evidence, doctors prescribe daily low-dose aspirin to people who have had a heart attack or stroke to help prevent that event from reoccurring. This is known as ‘secondary prevention’.
ASPREE was a clinical trial to determine whether low-dose aspirin helped older adults to live well for longer by delaying the onset of illnesses in the first place (called ‘primary prevention’) and whether the overall benefits outweighed the potential side effects of aspirin, such as bleeding.
The follow-up observational (no study medication) ASPREE-XT study, is the first large scale investigation into long-lasting effects of aspirin on cancer, dementia and frailty. To date there have been very few other studies that focus only on older adults.
Additionally, the ASPREE-XT study aims to identify demographic, health, genetic and environmental factors that affect thinking and memory, physical health and wellbeing in older people.
The ASPREE project will help discover how to maintain years of good quality life, with the findings relevant to communities today and to future generations around the world.
Because we cannot replace participants who leave the study, your ongoing involvement is critical to understand how we age. Thank you for sharing your health information and time – we are profoundly grateful for your contribution to this important research.
Participant Facts

19,114
research participants in Australia & the USA
From a total 19,114 participants, 16,703 healthy Australians aged 70+ enrolled in the ASPREE Trial between 2010 – 2014 (2,411 in the USA). Most heard about ASPREE from their GP.
9/10
enrolled in a sub-study in Australia
Participating involved donating blood and saliva samples for future research, through to MRI scans, hearing tests, retinal imaging and health questionnaires. View sub-studies here.
45%
from rural & regional south-east Australia
The ASPREE project has a high representation of participants from rural and regional areas in south eastern Australia. More than 5,000 participants are from regional Victoria.
More Information and FAQs for ASPREE participants
Click on a topic below
Update your details
What is the difference between ASPREE and ASPREE-XT?
FAQs ASPREE-XT study
FAQs ASPREE clinical trial
About Aspirin
Information documents
Participant newsletters
Can’t find what you are after?
Update your details
Moved house, new phone number, new GP?
Thank you for sharing your experience of ageing with the ASPREE team. Staying in touch with you is extremely important to the findings of the study. Everyone’s participation matters.
We need to be able to get in touch with you every six months. If you have moved, changed your phone number or email address, or GP since you last heard from us, please let us know.
- P: 1800 728 745 (toll free from a landline)
- E: aspree@monash.edu
Feedback
We value constructive feedback. Tell us what you think about your experience in the ASPREE project, using this website, attending ‘study update’ presentations, or suggestions for newsletters. Call 1800 728 745 (toll free from a landline) E: aspree@monash.edu
About ASPREE & ASPREE-XT
The ASPREE (ASPirin in Reducing Events in the Elderly) Project has two components:
- The ASPREE clinical trial (March 2010 – June 2017)
- The ASPREE-XT (eXTension) study (June 2017 – current)
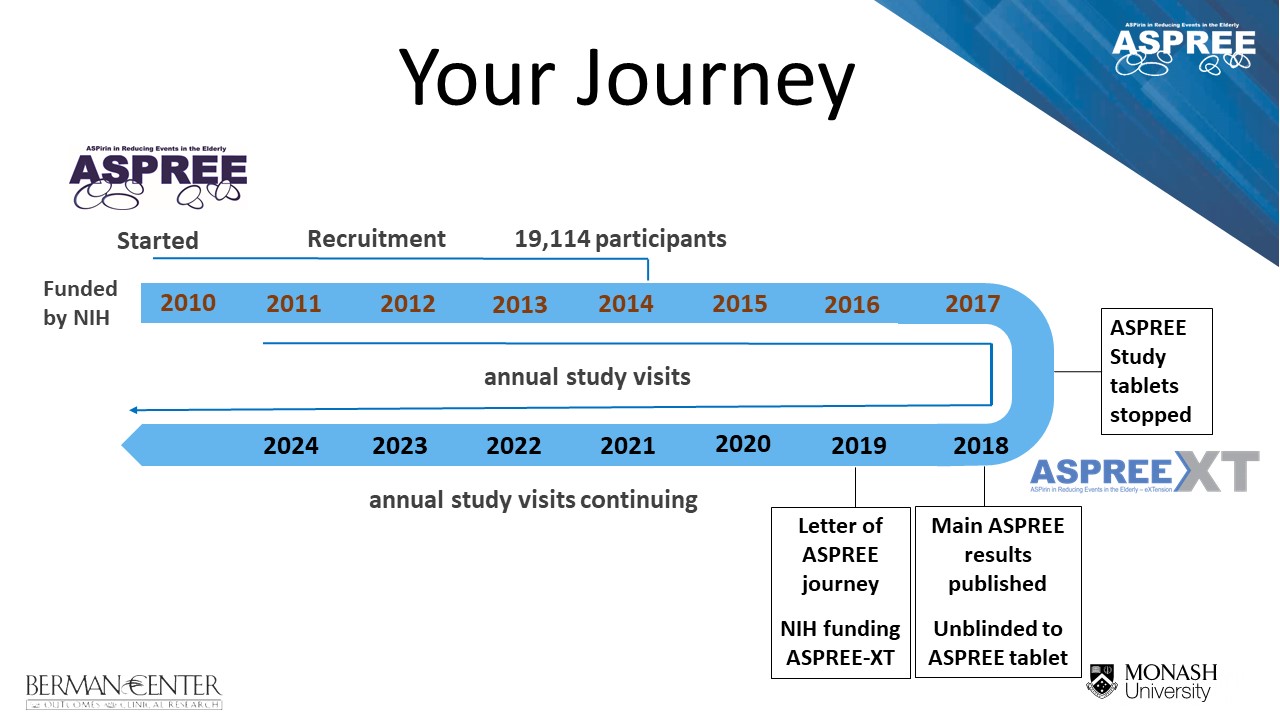
Above: An illustration of your journey through the ASPREE project, from enrolment in the ASPREE clinical trial during the recruitment period (2010 – 2014), to cessation of study tablets in June 2017, and progression to the current follow-up ASPREE-XT (eXTension) study.
ASPREE (ASPirin in Reducing Events in the Elderly) Clinical Trial:
March 2010 – June 2017
ASPREE was an international clinical trial to determine whether daily low-dose aspirin increased survival free of dementia or physical disability for healthy older people. It was a ‘gold standard’ trial – a randomised, double blind, placebo-controlled trial of daily low-dose aspirin (100mg) – and the first to investigate aspirin’s benefit versus risk in healthy older adults without a history of cardiovascular disease, dementia or significant physical disability. Fifty percent of participants were randomly assigned daily low-dose aspirin and fifty percent took a placebo.
The main trial results were published in three papers in the New England Journal of Medicine in September 2018. ASPREE found that over an average of 4.7 years, aspirin did not prolong life free of persistent physical disability or dementia (termed ‘disability free survival’) in 19,114 healthy community dwelling adults, most aged 70 years or older.
Aspirin did not significantly reduce the risk of heart attacks or strokes, but the risk of serious bleeding among the aspirin takers was increased compared to the placebo group, although the number of major bleeding events was small. A slightly higher death rate was observed in the aspirin group than the placebo group, mostly from cancer.
ASPREE’s findings provided doctors for the first time, with new scientific evidence of the benefits and risks of aspirin for disease prevention in healthy older adults. The National Institute on Aging (NIA) and Monash University announced the results around the world. The ASPREE team advised participants (and their GP) of their assigned study tablet and the trial’s results by mail around the time that the results were published. You can read more information about the trial findings here.
In Australia, ASPREE was conducted across south-eastern states and the ACT with participants represented from metropolitan, regional or rural areas, in collaboration with GPs.
The American Heart Association and the American College of Cardiology have already incorporated ASPREE trial findings into guidelines that help doctors know when (and when not to) prescribe aspirin to their patients. Many findings from the trial continue to be published today.
The ASPREE-XT (eXTension) Study:
June 2017 – 2024
ASPREE-XT (eXTension) is a follow-up study (no study medication) in ASPREE participants. It will determine whether participants in the aspirin group, compared to participants in the placebo group, have different health outcomes such as for cancer, dementia, frailty and other conditions of ageing, over the longer term.
Aspirin’s long-lasting effects on health needs to be studied further because diseases such as cancer and dementia develop over an extended period of time. The effect of aspirin on these diseases may not have been evident during the ASPREE trial.
In addition, ASPREE-XT will determine factors (such as those related to lifestyle, health behaviour, environment, genes, blood biomarkers (such as proteins) and many others) that may help predict good health and longevity, or factors that predispose to age-related diseases.
By sharing your health information with the ASPREE team, you continue to make a difference to how we understand health and ageing. We are very grateful to have you, our participants, without whom this new knowledge would not be possible.
ASPREE-XT is conducted similarly to the ASPREE trial, as outlined in the table below.
| ASPREE clinical trial | ASPREE-XT follow-up study | |
| Participants | Aged 70+ and healthy | ASPREE participants |
| In-person study visits | Annual | Annual |
| Phone calls | Every 3 months | Every 6 months |
| Study tablets | Yes (100 mg aspirin or placebo) | No study tablets |
| Access to medical records | Required | Required |
| Sub-studies | Yes | Yes |
| Study progress | Completed. Participants and GPs notified of assigned study tablet in 2018 | Currently underway |
| Leading research institutions |
Monash University (Australia) Berman Center for Outcomes & Clinical Research (USA) |
Monash University (Australia) Berman Center for Outcomes & Clinical Research (USA) |
FAQs ASPREE-XT follow up study
Q: What do you mean by long-lasting effects of aspirin?
A: Studies in middle aged adults suggest that potential benefits of daily low-dose aspirin for cancer prevention may not be apparent until some time after use. The reason for this is unknown. Some diseases, such as cancer and dementia can take several years to develop and the effect of aspirin on these diseases may not have been evident during the ASPREE trial.
ASPREE-XT is the first large scale study in the world to consider the long-lasting effects of aspirin on health in older adults.
Q: Why do you want to see me if I took the placebo tablet during the ASPREE trial?
A: ASPREE-XT will compare the health and well-being of participants in the aspirin group with participants in the placebo group over the longer term. We need participants from both groups to learn if aspirin has long lasting effects on health.
Q: My doctor put me on aspirin does that matter?
A: No, it does not matter which study tablet you took during the ASPREE trial, or whether you are or aren’t on aspirin now. In addition to studying the long-lasting effects of aspirin on health, ASPREE-XT will help to identify other factors that affect our health as we age.
Q: What happens if I develop a major health condition?
A: You can let us know of any personal health events by phone (1800 728 745), email (aspree@monash.edu) or at your in-person study visit.
Q: What happens if I lose my memory or physical ability?
A: We sincerely hope this doesn’t happen, but if it does, we will work around your personal circumstances and will tailor study activities accordingly. You may also like to nominate a ‘study partner’, who can assist with future participation in the study. Your experience of ageing, in good health or otherwise, makes an important contribution to the findings of the study.
FAQs About your Privacy
Q: Who will have access to my data?
A: Only a limited number of trained personnel have access to information which may identify you, such as your name, address, and telephone number. Every staff member and contractor has signed a confidentiality agreement as a condition of their employment. An example of a contractor is Monash’s mailing house service, which prints, folds and sends newsletters.
ASPREE and ASPREE-XT will never release your personal information to third parties, unless ordered to do so by a court order or by law.
Q: Can insurance companies, drug companies, employers, or my relatives find out about the results of research on my information?
A: No, they cannot. Your information is securely stored in a de-identified way. You have been allocated a study number which means information that can identify you, such as your name, address, or date of birth, is not recorded with your data.
Your personal information will NOT be released to any third parties, without your written consent, or unless ordered to do so by a court order or by law.
Q: How is my health information accessed?
A: Participants in the ASPREE project provide consent for bona fide research staff to access their health and prescribing information held by Medicare, the Pharmaceutical Benefits Scheme, and medical records held with your doctor, specialist or any hospital you may attend. This includes access to a Coroner’s report or other government health records.
Only medical information that is directly related to the study will be collected, de-identified, stored and analysed for the purposes of the research.
Q: What happens to information about me if I die while I am still a participant in the ASPREE-XT study?
A: We sincerely hope this doesn’t happen! In that unfortunate event, information collected during the course of the study would remain in the database and kept for research purposes.
Q: How long will my information be kept?
A: Indefinitely. Your information will be kept for research purposes. No individual will be identifiable in research publications.
Q: What if I decide at some point that I no longer want to be involved in ASPREE-XT?
A: Participation in ASPREE-XT and sub-studies is completely voluntary, however we cannot replace any participants who leave the study. We will work around your circumstances if you are experiencing difficulties. Please contact our team on 1800 728 745 (toll free from a landline) for a confidential discussion about any concerns you may have.
FAQs ASPREE Trial
Q: How long did the ASPREE trial run?
A: ASPREE started in March 2010 and study tablets were ceased in June 2017. The trial followed the health of participants for an average of 4.7 years. This means that people who joined the study in 2010 may have had seven in-person annual visits, while those who enrolled in 2014 will have had two or three. The ‘average’ time takes into consideration the time taken to recruit 19,114 people into the trial (2010 – 2014) and ensured that everyone finished in the same year.
Q: When did participants find out which study tablet they were assigned?
A: You and your GP were advised of your assigned tablet (100mg aspirin or matched placebo) around the same time as publication of the main ASPREE results in September 2018.
The ASPREE trial was a randomised double-blind study (some would say triple-blind) which ensured that for the duration of the trial, the ASPREE team members, you and your GP, did not know which group (placebo or aspirin) you belonged to. This was done to ensure that there was no bias in the data collected from participants, study staff or GPs that may have arisen if the medication assignments were known. Once the results were published, it was no longer necessary for you and your GP to remain blinded to your assigned study tablet.
Q: What happened at the end of the trial?
A: Three major ASPREE research papers were published in a prestigious, global medical journal, The New England Journal of Medicine in September 2018.
- Effect of Aspirin on Disability-free Survival in the Healthy Elderly (primary paper)
- Effect of Aspirin on All-Cause Mortality in the Healthy Elderly
- Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly
ASPREE findings were closely scrutinised by academic peers with relevant clinical expertise, specially selected by the high quality medical journal to review the trial results. ASPREE was the first large scale trial in the world to consider the impact of aspirin on disability-free survival (life free of physical disability or dementia).
More information about the results can be found on the associated media release and FAQs. Further analyses of the data collected in the ASPREE trial continue to be published in medical journals today.
All ASPREE participants were invited to be involved in an important follow-up health study, called ASPREE-XT (eXTension), to determine whether aspirin may have a long-lasting effect on health, such as prevention of cancer or dementia in older adults. ASPREE-XT, which commenced after the ASPREE study medication ceased in 2017, will also help identify other factors that may contribute to good health or frailty in later years. ASPREE-XT is the first large scale follow up study into long-lasting effects of aspirin and other factors affecting healthy ageing.
Q: What dose was used in the ASPREE trial and why?
A: ‘Low-dose’ 100mg of enteric-coated aspirin was trialled in ASPREE. The enteric coating on the aspirin acted to reduce abdominal discomfort. Half of ASPREE participants were randomly assigned low-dose aspirin, the other half a matching placebo.
The 100mg dose of aspirin has blood thinning (anti-platelet) actions with low side effects from bleeding. This dose is lower than that usually taken for pain relief and for reducing fever. It is the same dose prescribed for people who need it for secondary prevention, e.g. to prevent a second heart attack or stroke.
Q: Who was eligible to be in ASPREE?
A: Australian participants had to be:
- Aged 70 years or older (No one was too old!)
- Able-bodied and without dementia
- Without a history of a heart attack, stroke or a known bleeding risk
- Able to be randomly assigned to low-dose aspirin or a placebo tablet
- Able to undergo annual health measures at in-person visits
About Aspirin
Why is aspirin so special?
Aspirin is one of the most widely used and affordable medications in the world. It has been used in medical practice since the late 1890’s. Such is the importance of aspirin that it continues to be the subject of hundreds of published research studies each year.
How does aspirin work?
Aspirin has two main actions in the body:
- Stops blood platelets from clumping together to form a blood clot (‘blood thinner effect’) which can prolong the time it takes for bleeding to stop
- Reduces (at higher doses) the production in the body of chemicals called ‘prostaglandins’, thereby reducing inflammation, pain and fever
Uses of aspirin
- Low-dose aspirin (100 mg) is used after a first heart attack, angina or stroke to prevent a second or third similar event. This is known as ‘secondary prevention’.
- Higher doses of aspirin (300 – 500 mg) provides temporary relief of pain, inflammation and fever.
Side effects of aspirin
All medications have the potential to cause adverse effects, and aspirin is no exception. Aspirin belongs to a class of drugs called non-steroidal anti-inflammatory drugs (NSAID) which may interact with several other medications.
- Aspirin’s side effects are more commonly seen in older adults and in those receiving higher doses.
- Aspirin delays the ability of the blood to clot and block the leakage of blood from ruptured blood vessels.
- Aspirin reduces the effectiveness of the protective barrier in the stomach which may lead to bleeding from the stomach wall. This video explains how aspirin may cause bleeding in the stomach.
- More information about possible side-effects of low-dose aspirin and drug interactions is here and here.
It is important to talk to your doctor before starting daily low-dose aspirin even though you can buy it without a prescription.
History of Aspirin
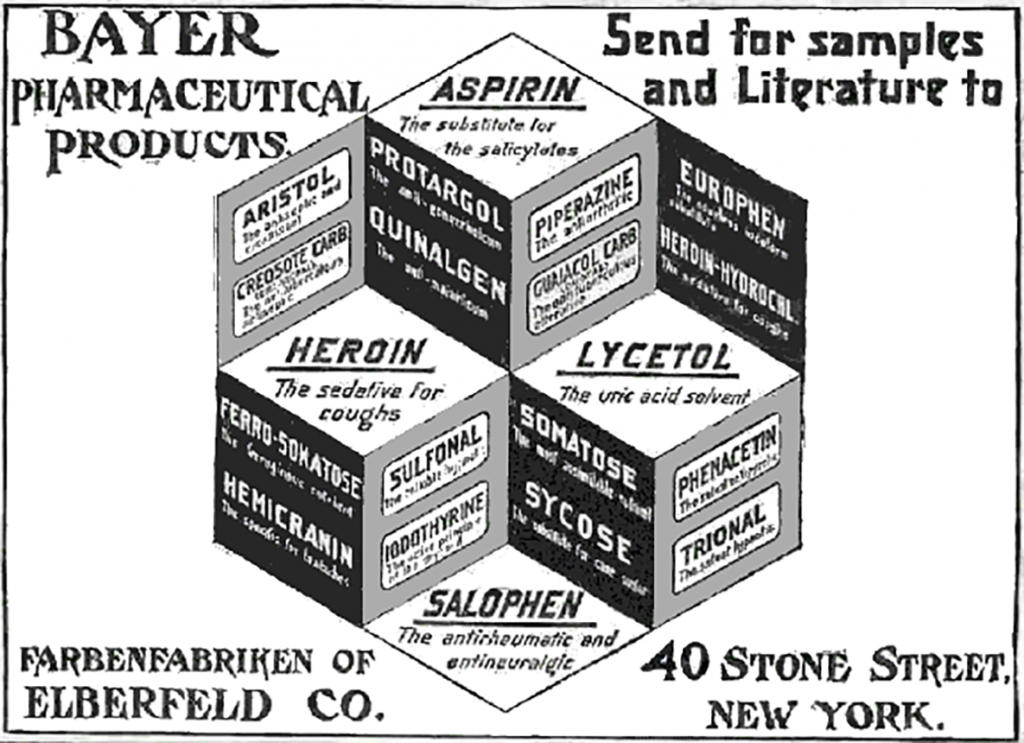 Above: Until the early 1900’s aspirin was once marketed alongside Heroin and Cocaine
Above: Until the early 1900’s aspirin was once marketed alongside Heroin and Cocaine
Aspirin in the Ancient World
- Aspirin was first produced in Germany 110 years ago, but its natural form, salicylic acid, found in plants (i.e. the willow tree and myrtle) has been used for thousands of years.
- As early as 3000 BC, the ancient Egyptians used willow bark and myrtle to reduce pain and fever
- By 30 AD, Greek and Roman physicians recommended the use of willow leaf to treat inflammation.
A Victorian Era Discovery
- Salicylic acid was associated with a bitter taste and often induced upset stomachs and vomiting.
- In 1897, Felix Hoffmann (a chemist working for Bayer Company in Germany) synthesised acetyl salicylic acid which largely reduced the stomach irritation caused by salicylate alone.
- Two years later, on March 6, 1899, aspirin was trademarked under the Imperial Office of Berlin.
- When war broke out in 1914, the Allies lost their source of aspirin and so they offered large prizes for anyone who could make aspirin. The Nicholas brothers from Melbourne were among the first to synthesise commercial quantities, which they called Aspro.
An Aspirin a day
- The ability of aspirin to prevent heart attacks and stroke was first proposed in the 1940s when doctors observed that children who were given aspirin-laced chewing gum to relieve pain after a tonsillectomy bled more than those who did not have the gum. It was reasoned that if aspirin caused bleeding it could prevent clotting, the cause of heart attacks.
- Because these initial studies were published in obscure journals, the recommendation to take an aspirin a day to prevent recurrent heart attacks was not adopted. It wasn’t until the 1970s when adequately controlled trials established that this was true, that doctors routinely started recommending aspirin for secondary prevention.
Aspirin Today
- Aspirin is now available in over 80 countries.
- It is regarded as the most successful non-prescription medicine of all time.
- Aspirin was one of the very first drugs to be made available in tablet-form.
- It is now a standard worldwide remedy for pain, inflammation and fever.
Watch a video on the chemical structure of aspirin here.
Fun facts about Aspirin
- The melting point of aspirin (acetylsalicylic acid, ASA) is 135 °C.
- In 1950, aspirin was entered into the Guinness Book of Records as the highest-selling drug product.
- Approximately 25,000 scientific articles about aspirin and a projected one trillion aspirin pills have been consumed since it arrived on the market.
- Approximately 40,000 tonne of aspirin is produced annually worldwide.
- Adding aspirin to water in a vase will make cut flowers last longer. The effect is attributable to salicylic acid, which as a messenger substance plays an important role in plants’ survival.
- Before 1904, Bayer sold aspirin to pharmacies as a fine, white powder in bottles.
- After becoming world famous for developing aspirin, Felix Hoffmann disappeared completely from the public eye. On retiring in 1928, he emigrated to Switzerland where he lived in seclusion until his death in 1946.
- In 2002, Felix Hoffmann was inducted into the US National Inventors Hall of Fame.
- After aspirin was developed in 1897, it was around 70 years before British scientist Professor Sir John R. Vane made the Nobel Prize winning discovery that it worked by inhibiting prostaglandins (pain messenger chemicals).
- Aspirin went into space, as part of the on-board medicine kit on all of the Apollo rockets that NASA sent to the moon – both the orbital lunar flights in 1968/69 and the seven moon landings from 1969 to 1972 (Apollo 11 to Apollo 17).
- Salicylic acid is found in certain vegetables, fruits, nuts, seeds and herbs in various amounts. Acetyl salicylic acid in aspirin, is a modified version of salicylic.
Sources and further reading: History of Aspirin, Pharmaceutical Journal, Aspirin timeline, Wikipedia
Information documents
ASPREE Brochures
Study Protocols
A study protocol is the reference document for a research project. The protocol documents the background to the study, the trial design, objectives, hypothesis, endpoints, patient well-being, study visit measurements, analysis and adherence to ethical regulations. Study protocols must be approved by a Human Research Ethics Committee (HREC) before a study can legally commence in Australia.
Newsletters
This biannual publication helps keep you up to date on the ASPREE project – from study progress, achievements and findings, through to news stories, reminders and snippets. Submissions to future newsletters (limited to 100 words is ideal) and/or story suggestions are welcome! Send through your suggestions to aspree@monash.edu
View past editions of the ASPREE participant newsletter:
- Participant newsletter_autumn 2024
- Participant newsletter_spring 2023
- Participant newsletter_summer_2022-2023
- Participant_newsletter_winter&spring_2022
- Participant_newsletter_summer_2021-2022
- Participant_newsletter_winter_2021
- Participant_newsletter_summer_2020-2021
- Participant_newsletter_winter_2020
- Participant_newsletter_summer_2019-2020
- Participant_newsletter_summer&autumn_2018-2019
- Participant-newsletter_summer_2017-2018
- Participant_newsletter_winter&spring_2017
- Participant_newsletter_winter&spring_2016
- Participant_newsletter_summer_2015-2016
- Participant_newsletter_autumn&winter_2015
- Participant_newsletter_spring&summer_2014 and a letter of thanks from Prof John McNeil
- Participant_newsletter_spring&summer_2013-2014
- Participant_newsletter_autumn&winter 2013
- Participant_newsletter_spring&summer_2012-2013
- Participant_newsletter_autumn_2012
- Participant_newsletter_spring&summer_2011-2012
- Participant_newsletter_winter_2011
- Participant_newsletter_spring&summer_2010-2011
- Participant_newsletter_winter_2010
- Participant_newsletter_autumn_2010
We are very happy to answer your questions. Contact the ASPREE team:
- P: 1800 728 745 (toll free from a landline)
- E: aspree@monash.edu
Page updated: 1 August 2023
NEWS FOR PARTICIPANTS
Walking for transport at least once a week may help some older people live longer: ASPREE study
A new analysis of ASPREE data found that older adults who walked for transport instead of taking a car, at least once a week, lived longer than those who didn’t. This observational study in older adults looked at transport-related walking, which is walking for a specific purpose, such as to a medical appointment or to shop, instead of using motorised transport. Researchers found walking for transport at least once a week linked to a 25% lower risk of mortality compared to those who never walked for transport purposes.
Leisure activities that may reduce dementia risk – ASPREE analysis
In a study of leisure activities undertaken by ASPREE participants, using computers, writing, and...
Aspirin and anaemia risk in older adults
A new analysis of ASPREE trial data has found that prolonged daily aspirin use increases the risk of anaemia in some older adults. Findings from ASPREE-Anaemia, a sub-study of the ASPREE trial, may help GPs identify older patients at higher risk of anaemia and who may benefit from regular monitoring for development of the condition.