FOR PARTICIPANTS
ASPREE
- The ASPREE Clinical Trial (completed)
- The follow-up ASPREE-XT Study (current)
→ Update your details
→ About Participants
→ FAQs, newsletters and more
→ Latest news
→ Contact us
The follow-up observational (no study medication) ASPREE-XT study, is the first large scale investigation into long-lasting effects of aspirin on cancer, dementia and frailty. To date, very few studies focus solely on older adults.
Additionally, the ASPREE-XT study aims to identify demographic, health, genetic, and environmental factors that affect thinking and memory, physical health and wellbeing in older people.
The ASPREE project will help discover how to maintain years of good quality life, with the findings relevant to communities today and to future generations around the world.
Because we cannot replace participants who leave the study, your ongoing involvement is critical to understand how we age. Thank you for sharing your health information and time; we are extremely grateful for your continued contribution to this important research.
Participant Facts

2,411
United States ASPREE participants

80%
100%
More Information and FAQs for ASPREE participants
Click on a topic below
Update your details
Have you moved? Do you have a new phone number? A new Primary Care Provider (PCP)?
Thank you for sharing your experience of aging with the ASPREE team. Staying in touch with you is extremely important to the findings of the study and everyone’s participation matters.
We need to be able to get in touch with you every six months. If you have moved, changed your phone number, email address, or your PCP since you last heard from us, please contact your study staff to update your contact details. Click here to find the phone number of your ASPREE-XT study site.
Feedback
We value your feedback. Please tell us what you think about your experience in the ASPREE project, using this website, attending ‘study update’ presentations, or suggestions for newsletters. Click here to find the phone number of your ASPREE-XT study site.
About ASPREE and ASPREE-XT
The ASPREE (ASPirin in Reducing Events in the Elderly) project has two components:
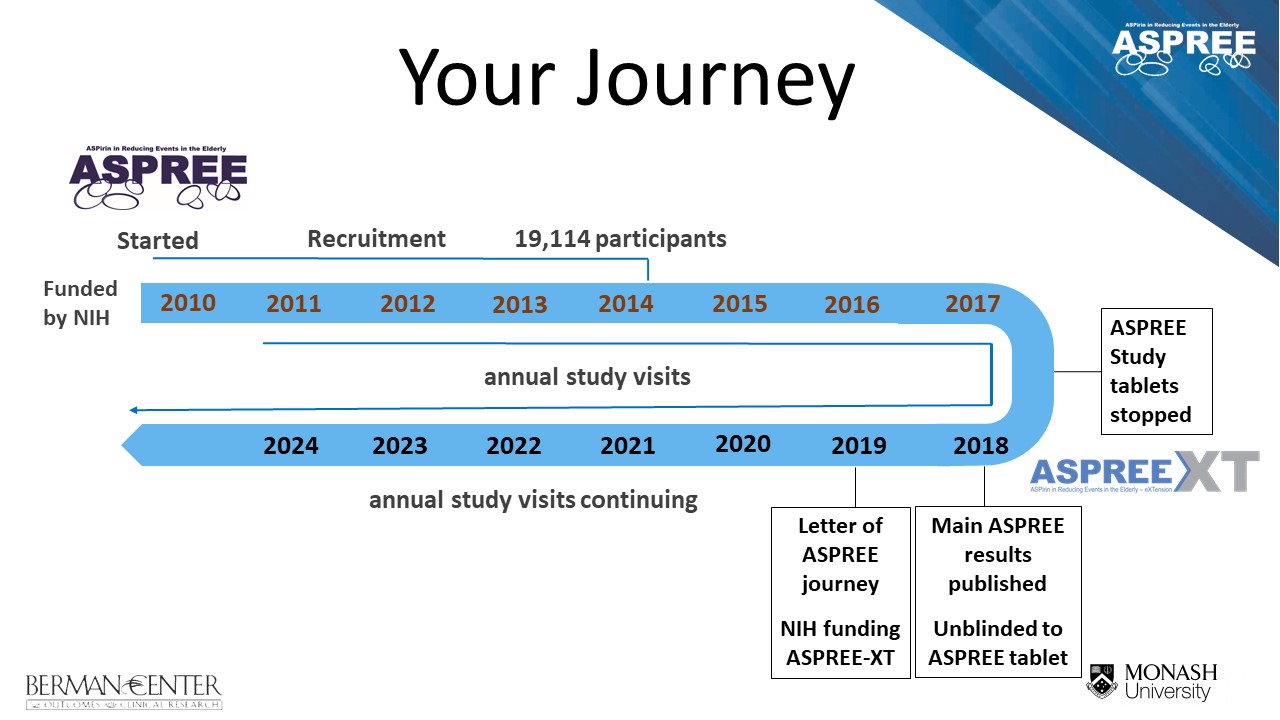
- The ASPREE clinical trial (March 2010 – June 2017)
- The ASPREE-XT (eXTension) study (June 2017 – current)
ASPREE (ASPirin in Reducing Events in the Elderly) Clinical Trial:
March 2010 – June 2017
ASPREE was an international clinical trial to determine whether daily low-dose aspirin increased survival free of dementia or physical disability for healthy older people. It was a ‘gold standard’ trial – a randomized, double blind, placebo-controlled trial of daily low-dose aspirin (100mg) – and the first to investigate aspirin’s benefit versus risk in healthy older people without a history of cardiovascular disease, dementia, or significant physical disability. 50% of participants were randomly assigned daily, low-dose aspirin and 50% took a placebo.
The main trial results were published in three papers in the New England Journal of Medicine in September 2018. ASPREE found that over an average of 4.7 years, aspirin did not prolong life free of persistent physical disability or dementia (termed ‘disability free survival’) in 19,114 healthy community dwelling adults, most aged 70 years or older.
Aspirin did not significantly reduce the risk of heart attacks or strokes, but the risk of serious bleeding among the aspirin takers was increased compared to the placebo group, although the number of major bleeding events was small. A slightly higher death rate was observed in the aspirin group than the placebo group, mostly from cancer.
ASPREE’s findings provided doctors for the first time, with new scientific evidence of the benefits and risks of aspirin for disease prevention in healthy older adults. The National Institute on Aging (NIA) announced the results around the world. The ASPREE team advised participants of their assigned study tablet and the trial’s results by mail around the time that the results were published. You can read more information about the trial findings here.
The American Heart Association and the American College of Cardiology have already incorporated ASPREE trial findings into guidelines that help doctors know when (and when not to) prescribe aspirin to their patients. Many findings from the trial continue to be published today.
The ASPREE-XT (eXTension) Study:
June 2017 – 2024
ASPREE-XT (eXTension) is a follow-up study (no study medication) in ASPREE participants. It will determine whether participants in the aspirin group, compared to participants in the placebo group, have different health outcomes such as for cancer, dementia, frailty and other conditions of aging, over the longer term.
Aspirin’s long-lasting effects on health needs to be studied further because diseases such as cancer and dementia develop over an extended period of time. The effect of aspirin on these diseases may not have been evident during the ASPREE trial.
In addition, ASPREE-XT will determine factors (such as those related to lifestyle, health behavior, environment, genes, blood biomarkers (such as proteins) and many others) that may help predict good health and longevity, or factors that predispose to age-related diseases.
By sharing your health information with the ASPREE team, you continue to make a difference on how we understand health and aging. We are very grateful to have you, our participants, without whom this new information would not be possible.
| ASPREE clinical trial | ASPREE-XT follow-up study | |
| Participants | Aged 65+ and healthy | ASPREE participants |
| In-person Study Visits | Annual | Annual |
| Phone Calls | Every 3 months | Every 6 months |
| Study Tablets | Yes (100 mg aspirin or placebo) | No study tablets |
| Access to Medical Records | Required | Required |
| Sub-studies | Yes | Yes |
| Study Progress | Completed. Participants and GPs notified of assigned study tablet in 2018 | Currently underway |
| Leading Research Institutions |
Berman Center for Outcomes & Clinical Research (USA) Monash University (Australia) |
Berman Center for Outcomes & Clinical Research (USA) Monash University (Australia) |
FAQs ASPREE-XT follow-up study
Q: What do you mean by long-lasting effects of aspirin?
A: Studies in middle-aged adults suggest that potential benefits of daily, low-dose aspirin for cancer prevention may not be apparent until sometime after use. The reason for this is quite simply, unknown. Some diseases, such as cancer and dementia can take several years to develop. and the effect of aspirin on these diseases may not have been evident during the ASPREE trial.
ASPREE-XT is the first large scale study in the world to consider the long-lasting effects of aspirin on health in older adults.
Q: Why do you want to see me if I took the placebo tablet during the ASPREE trial?
A: ASPREE-XT will compare the health and wellbeing of participants in the aspirin group with participants in the placebo group over the longer term. We need participants from both groups to learn if aspirin has long-lasting effects on health.
Q: My doctor put me on aspirin does that matter?
A: No, it does not matter which study tablet you took during the ASPREE trial, or whether you are, or are not on aspirin now. In addition to studying the long-lasting effects of aspirin on health, ASPREE-XT will help to identify other factors that affect our health as we age.
Q: What happens if I develop a major health condition?
A: Please let us know if you have any personal health events either by phone, email, or at your in-person study visit.
Q: What happens if I lose my memory or physical ability?
A: : We sincerely hope this does not happen, but if it does, we will work around your personal circumstances and will accommodate your study activities accordingly. You can also choose to nominate a ‘study partner’ to assist you with future participation in the study. Your experience of aging, in good health or otherwise, makes an important contribution to the findings of the study.
About your Privacy
- All participants are allocated an ID number in place of personal information such as name and address. Information we collect is entered into a de-identified database via the secure ASPREE Data Entry website.
- Access to this web-based portal is only made available to registered study staff via a password-protected and secure login.
- Data held at the ASPREE Study Center is locked within a secure environment both physically (paper files) and electronically.
- Data collection, storage, and management is centralized at the ASPREE Clinical Trial Center in Melbourne, Australia, allowing us to track the location of files at any time.
- All ASPREE and ASPREE-XT study staff have been trained and understand the importance of confidentiality and privacy. In addition, all staff have signed confidentiality agreements as part of their employment with the study.
FAQs ASPREE Trial
Q: How long did the ASPREE trial run?
A: ASPREE started in March 2010 and study tablets were ceased in June 2017. The trial followed the health of participants for an average of 4.7 years. This means that people who joined the study in 2010 may have had seven in-person annual visits, while those who enrolled in 2014 will have had two or three. The ‘average’ time takes into consideration the time taken to recruit 19,114 people into the trial (2010 – 2014) and ensured that everyone finished in the same year.
Q: When did participants find out which study tablet they were assigned?
A: You and your Primary Care Provider (PCP) were advised of your assigned tablet (100 mg aspirin or matched placebo) around the same time as the publication of the main ASPREE results in September 2018.
The ASPREE trial was a randomized, double-blind study, which ensured that for the duration of the trial, the ASPREE team members, you, and your Primary Care Provider (PCP), did not know which group (placebo or aspirin) you belonged to. This was done to ensure that there was no bias in the data collected from participants, study staff or PCPs that may have arisen if the medication assignments were known. Once the results were published, it was no longer necessary for you and your PCP to remain blinded to your assigned study tablet.
Q: What happened at the end of the trial?
A: Three major ASPREE research papers were published in a prestigious, global medical journal, The New England Journal of Medicine in September 2018.
- Effect of Aspirin on Disability-free Survival in the Healthy Elderly (primary paper)
- Effect of Aspirin on All-Cause Mortality in the Healthy Elderly
- Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly
ASPREE findings were closely scrutinised by academic peers with relevant clinical expertise, specially selected by the high quality medical journal to review the trial results. ASPREE was the first large scale trial in the world to consider the impact of aspirin on disability-free survival (life free of physical disability or dementia).
More information about the results can be found on the associated media release and FAQs. Further analyses of the data collected in the ASPREE trial continue to be published in medical journals today.
All ASPREE participants were invited to be involved in an important follow-up health study, called ASPREE-XT (eXTension), to determine whether aspirin may have a long-lasting effect on health, such as prevention of cancer or dementia in older adults. ASPREE-XT, which commenced after the ASPREE study medication ceased in 2017, will also help identify other factors that may contribute to good health or frailty in later years. ASPREE-XT is the first large scale follow up study into long-lasting effects of aspirin and other factors affecting healthy aging.
Q: What dose was used in the ASPREE trial and why?
A: Low-dose, 100 mg of enteric-coated aspirin was tested in ASPREE. The enteric coating on the aspirin acted to reduce abdominal discomfort. Half of ASPREE participants were randomly assigned low-dose aspirin, the other half, a matching placebo.
The 100 mg dose of aspirin has blood thinning (anti-platelet) actions with low side effects from bleeding. This dose is lower than that usually taken for pain relief and for reducing fever. It is the same dose prescribed for people who need it for secondary prevention (e.g. to prevent a second heart attack or stroke).
About Aspirin
Why is aspirin so special?
Aspirin is one of the most widely used and affordable medications in the world. It has been used in medical practice since the late 1890’s. Such is the importance of aspirin that it continues to be the subject of hundreds of published research studies each year.
How does aspirin work?
Aspirin has two main actions in the body:
- Prevents blood platelets from clumping together to form a blood clot (‘blood thinner effect’). which can prolong the time it takes for bleeding to stop.
- Higher doses of aspirin reduces the production in the body of chemicals called ‘prostaglandins’, thereby reducing inflammation, pain, and fever.
Uses of aspirin
- Low-dose aspirin (100 mg) is used after a first heart attack, angina or stroke to prevent a second or third similar event. This is known as ‘secondary prevention.’
- Higher doses of aspirin (300 – 500 mg) provides temporary relief of pain, inflammation and fever.
Side effects of aspirin
All medications have the potential to cause adverse effects, and aspirin is no exception. Aspirin belongs to a class of drugs called non-steroidal anti-inflammatory drugs (NSAID) which may interact with several other medications.
- Aspirin’s side effects are more commonly seen in older adults and in those receiving higher doses.
- Aspirin delays the ability of the blood to clot and block the leakage of blood from ruptured blood vessels.
- Aspirin reduces the effectiveness of the protective barrier in the stomach which may lead to bleeding from the stomach wall. This video explains how aspirin may cause bleeding in the stomach.
- More information about possible side-effects of low-dose aspirin and drug interactions is here and here.
Note: it is important to talk to your Primary Care Provider before starting daily low-dose aspirin even though you can buy it without a prescription.
Fun facts about Aspirin
- The melting point of aspirin (acetylsalicylic acid, ASA) is 275 °F.
- In 1950, aspirin was entered into the Guinness Book of Records as the highest-selling drug product.
- Approximately 25,000 scientific articles about aspirin and a projected one trillion aspirin pills have been consumed since it arrived on the market.
- Approximately 40,000 tons of aspirin are produced annually worldwide.
- Adding aspirin to water in a vase will make cut flowers last longer. The effect is attributable to salicylic acid, which as a messenger substance plays an important role in plants’ survival.
- Before 1904, Bayer sold aspirin to pharmacies as a fine, white powder in bottles.
- After becoming world famous for developing aspirin, Felix Hoffmann disappeared completely from the public eye. After retiring in 1928, he moved to Switzerland where he lived in seclusion until his death in 1946.
- In 2002, Felix Hoffmann was inducted into the US National Inventors Hall of Fame.
- After aspirin was developed in 1897, it was around 70 years before British scientist Professor Sir John R. Vane made the Nobel Prize winning discovery that it worked by inhibiting prostaglandins (pain messenger chemicals).
- Aspirin went into space, as part of the on-board medicine kit on all of the Apollo rockets that NASA sent to the moon – both the orbital lunar flights in 1968/69 and the seven moon landings from 1969 to 1972 (Apollo 11 to Apollo 17).
- Salicylic acid is found in certain vegetables, fruits, nuts, seeds and herbs in various amounts. Acetyl salicylic acid in aspirin, is a modified version of salicylic acid.
Sources and further reading: History of Aspirin, Pharmaceutical Journal, Aspirin timeline, Wikipedia
Information documents
ASPREE Brochures
Newsletters
The newsletters help keep you and the general community up-to-date on the ASPREE project – from study progress to news stories, and reminders. If you have any suggestions or ideas for future newsletter content, please email Brenda Kirpach at: BKirpach@bermancenter.org Thank you!
View past editions of the ASPREE participant newsletter:
- ASPREE-XT Newsletter Summer Vol 22, 2024
- ASPREE-XT Newsletter Winter Vol 21, 2024
- ASPREE-XT Newsletter Summer Vol 20, 2023
- ASPREE-XT Newsletter Winter Vol 19, 2023
- ASPREE-XT Newsletter Summer Vol 18, 2022
- ASPREE-XT Newsletter Winter Vol 17, 2021
- ASPREE-XT Newsletter Winter Vol 16, 2021
- ASPREE-XT Newsletter Spring Vol 15, 2020
- ASPREE Newsletter Summer Vol 14, 2017
- ASPREE Newsletter Winter Vol 13, 2017
- ASPREE Newsletter Summer Vol 12, 2016
- ASPREE Newsletter Winter Vol 11, 2016
- ASPREE Newsletter Summer Vol 10, 2015
- ASPREE Newsletter Winter Vol 9, 2014
- ASPREE Newsletter Summer Vol 8, 2014
Page updated: August 15, 2023
NEWS FOR PARTICIPANTS
Aspirin and anemia risk in older adults
Low-dose aspirin may increase anemia risk in healthy older adults: study A new study analysing data from the landmark ASPREE trial has found that prolonged daily aspirin use increases the risk of anemia by 20% in some older adults, mostly aged over 70 years (65...
Findings continue to impact aspirin guidelines
U.S. medical expert group latest to cite ASPREE results in guidelines Major findings from the ASPREE trial have been adopted into influential US Preventive Services Taskforce (USPSTF) new medical aspirin guidelines. The USPSTF recently announced a new recommendation...
Blood pressure variability associated with increased risk of dementia, especially in men
New analysis of ASPREE data reveals high long term blood pressure variability (BPV) in older adults, particularly men, is associated with an increased risk of dementia and cognitive decline